Magnetic Resonance Imaging in proton therapy

Maryam Moteabbed, PhD
Massachusetts General Hospital, Harvard Medical School, Boston, MA. USA![]()
Proton therapy enables significantly improved therapeutic ratio compared to conventional photon-based radiotherapy for many cancer types. Nevertheless, full realization of the potential clinical benefits of this modality is currently hindered by the relatively high susceptibility of proton dose to uncertainties, e.g. setup, interfractional anatomy variations, motion and range uncertainty. Large target margins are often needed to compensate for the impact of such uncertainties on the delivered dose. Onboard volumetric image guidance strategies currently available on photon-based linear accelerators are largely lacking in proton therapy, impeding further significant improvements in treatment planning and delivery.
The integration of onboard Magnetic Resonance Imaging (MRI) with linear accelerators (i.e. MR-linac) is the most recent advancement in image-guided radiotherapy, which has already shown significant improvement in clinical treatment outcomes, e.g. by enabling dose escalation for pancreatic cancer treatments [1]. Given the higher dosimetric sensitivity, proton therapy would more greatly benefit from MRI guidance, through online delivery monitoring and treatment adaptations. Combining the superior soft tissue contrast and real-time imaging capabilities of MRI with the inherent dosimetric advantages of protons will provide a compelling opportunity to fully exploit the unparalleled clinical potential of proton therapy, and an ideal setting to enhance treatment quality and consequently quality of life and survival for many cancer patients.
A true MR-integrated proton therapy system could offer superior accuracy compared with offline and in-room imaging options, especially for moving or hard to visualize tumors. A recent study has shown that margin reduction enabled by a hypothetical MR-integrated proton therapy could significantly decrease the normal tissue complication probability compared with MR-linac, offline MRI-guided proton therapy and online Cone beam CT-guided proton therapy for liver tumors (Fig. 1) [2]. The primary concern with such integrated system, however, is the impact of the MR magnetic field on the proton beam and planned dose, which has been shown to be addressable through delivery adjustments and/or inverse optimization [3,4]. Other challenges are related to proton dose calculation on MR images and adapting the dose deposition while controlling the beam range and deflection in real-time. A pre-clinical prototype system constructed in Dresden, Germany is currently exploring the mutual interactions between the two systems and has found promising results [5].
Given the current ongoing efforts, the prospect of the first clinical MR-integrated proton therapy prototype system is on the horizon [6]. In the meantime, however, a dedicated MRI scanner in the proton therapy suite to facilitate offline treatment adaptations, could play an important role in improving treatment efficacy and expanding the application of this promising modality to other clinical scenarios and cancer sites.
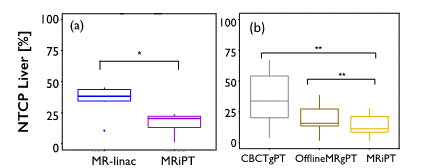
Figure 1. Significant decrease of normal tissue complication probability (NTCP) of the uninvolved liver with MR-integrated proton therapy (MRiPT), compared with (a) MR-linac, and (b) online cone beam CT and offline MRI-guided proton therapy [2].
References
1. Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123-2132.
2. Moteabbed M, Smeets J, Hong TS, et al. Toward MR-integrated proton therapy: modeling the potential benefits for liver tumors. Phys Med Biol. 2021:66(19), 195004.
3. Moteabbed M, Schuemann J, Paganetti H. Dosimetric feasibility of real-time MRI-guided proton therapy. Med Phys 2014;41:111713.
4. Hartman J, Kontaxis C, Bol GH, Frank SJ, Lagendijk JJW, Vulpen M van, et al. Dosimetric feasibility of intensity modulated proton therapy in a transverse magnetic field of 1.5 T. Phys Med Biol 2015;60:5955–5969.
5. Gantz S, Hietschold V, Hoffmann AL. Characterization of magnetic interference and image artefacts during simultaneous in-beam MR imaging and proton pencil beam scanning. Phys Med Biol. 2020;65(21):215014.
6. Hoffmann AL, Oborn B, Moteabbed M, et al. MR-guided proton therapy: a review and a preview. Radiat Oncol 2020;15(1):129.

