Virtual imaging trials

Ehsan Samei
Duke University, NC, United States![]()
Virtual imaging trials
Scientific advances in medicine have always faced a significant challenge: an inability to conduct experiments in vivo. Certain types of experimentation are allowed, namely in petri dishes, in animals, and in humans through so called clinical trials. Human experimentations through clinical trials have always been most definitive, but they have also been highly limited due to their extreme adherence to ethical standards, justifiably so, as well as their tremendous expense.
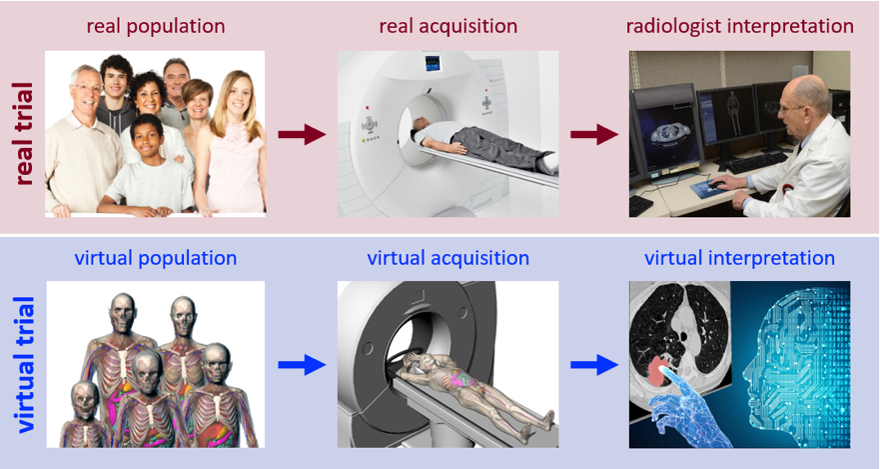
Fig. 1. Conducting a clinical imaging trial virtually. Imaging process (top) emulated by virtual imaging trial (bottom).
For those deemed feasible and fundable, the timing of the results can also be far out of step with the pace of innovation and with what needs to be known quicky, to avoid “costly” delays in the care of our patients. Consequently, regulatory approval of new innovations always takes longer than one wishes to see, and most of their design and use choices remain sub- or un-optimized. This is particularly the case in the development and use of advanced imaging and therapy applications, when the rate of their innovations and the multiplicity of their design and use choices far outweigh our ability to try them out through clinical trials.
In the last few years, virtual clinical trials have emerged as a new experimental paradigm to overcome the limitation of clinical trials [1-2]. In a manner analogous to clinical trials, Fig. 1, virtual clinical trials effectually provide computerized patients (so called digital twins)[3-4], computational models of technology being evaluated [5-6], and computational physicians to interpret the outcome of the intervention [7-10]. Virtual Imaging Trials (VIT) offers a practical solution to the in vivo experimentation challenge in such a way that can both keep up with the pace of innovations in medicine and also ensure rigorous scientific control over trial parameters. To be effective, however, a VIT requires patient models to realistically represent the human anatomy inside and out, imaging models that accurately simulate the precise details of the manufacturer-specific imaging processes, and interpretation models that reflect both human and machine perception of medical images.
The NIH/NIBIB recently granted Duke University a Program Project grant to develop a Center for Virtual Imaging Trials. Initiated and hosted by Carl E. Ravin Advanced Imaging Laboratories. The Center joins 24 other such resource centers in the US to develop and provide a virtual platform to assess the clinical performance of medical imaging systems from design to use. The Center is composed of three developmental, a training and dissemination, and 17 collaborating projects in academia, industry, and government, including international partners. While in its initial phase the Center will be focusing on CT imaging, the concept of virtual trials has broad implications beyond CT to other imaging modalities and to medicine at large.
References:
- Samei E, Kinahan P, Nishikawa R, Maidment A. Virtual clinical trials: why and what. (Invited editorial). Journal of Medical Imaging 7(4): 042801, 2020.
- Abadi E, Segars WP, Tsui BMW, Kinahan PE, Bottenus N, Frangi AF, Maidment A, Lo JY, Samei E. Virtual clinical trials in medical imaging: a review. Journal of Medical Imaging 7(4): 042805, 2020.
- Segars W, Sturgeon G, Mendonca S, Grimes J and Tsui BM. 4D XCAT phantom for multimodality imaging research. Medical Physics 37: 4902-4915, 2010.
- Segars WP, Norris H, Sturgeon GM, Zhang Y, Bond J, Minhas A, Tward DJ, Ratnanather TJT, Miller MI, Frush DP, Samei E. The development of a population of 4D pediatric XCAT phantoms for imaging research and optimization. Medical Physics 42(8): 4719-4726, 2015.
- Abadi E, Harrawood B, Sharma S, Kapadia A, Segars WP, Samei E. DukeSim: A realistic, rapid, and scanner-specific simulation framework in computed tomography. IEEE Trans Med Imaging 38(6): 1457-1465, 2019.
- Sharma S, Kapadia A, Fu W, Abadi E, Segars WP, Samei E. A real-time Monte Carlo tool for individualized dose estimations in clinical CT. Physics in Medicine and Biology 64(21), 215020, 2019.
- Solomon J, Samei E. Correlation between human detection accuracy and observer model-based image quality metrics in CT. Journal of Medical Imaging 3(3): 035506, 2016.
- Smith TB, Solomon J, Samei E. Estimating detectability index in vivo: development and validation of an automated methodology. Journal of Medical Imaging 5(3): 031403, 2017.
- Hoye J, Solomon BJ, Sauer TJ, Samei E. Quantification of minimum detectable difference in radiomics features across lesions and CT imaging conditions. Academic Radiology (online release 8/22/19, in print 2021).
- Samei E, Richards T, Segars WP, Daubert MA, Ivanov A, Rubin GD, Douglas PS, Udo Hoffmann U. A task-dependent estimability index to assess the quality of cardiac CTA to quantify coronary stenosis. Journal of Medical Imaging 8(1): 013501, 2021.

